Our immune systems are incredible. Every day, we breathe in millions of pathogens, such as bacteria and viruses. Yet we aren’t constantly ill. That’s because our immune system fights off thousands of threats every single day.
The immune system works by noticing things that don’t belong in your body. One part of the immune system is ‘innate’, which gives a rapid attack against pathogens such as bacteria or viruses.
The other clever part of our immune system is ‘adaptive’, where specialised immune cells can recognise and target specific antigens (foreign substances in the body). These might be things like pieces of pollen or proteins from bacteria and viruses. There are a few different types of immune cells that can recognise these antigens, and they have a variety of ways to remove the problem.
The immune system also plays a role in making sure our cells are healthy. It finds and kills infected or mutated cells, such as early cancer cells, every day. However, cancer cells can make it hard for the immune system to find them. Some cancer cells can hide or trick the immune system, eventually leading to the development of cancer. At that point, the immune system can’t handle the problem alone, and doctors need to step in.
There are a variety of cancer treatments available, and a lot of research is going on into finding better ones. In particular, there’s a lot of buzz about immunotherapy, which is a type of cancer treatment that helps the body’s own immune system fight cancer.
Immunotherapy isn’t an especially modern idea though. The first recorded case of immunotherapy being used to treat cancer was by Dr William B Coley in 1891 – over 130 years ago. Now known as the ‘Father of Immunotherapy’, William purposely infected a terminal bone cancer patient with carefully chosen bacteria. To his joy, the patient survived another eight years.
It wasn’t until 50 years later that the era of reliable and trustworthy immunotherapy really began. Since then, there has been an explosion in the ways doctors can treat cancer. So, let’s take a look at some of these fascinating ways of treating cancer with immunotherapy.
Cytokine therapy
Cytokines are small proteins that immune cells use to communicate with each other. They’re really important for coordinating the complex interactions between all of the different immune cells needed to combat cancer. Cytokines can activate cancer-fighting cells in different places in the body, and make it easier for immune cells to recognise and attack cancer cells.
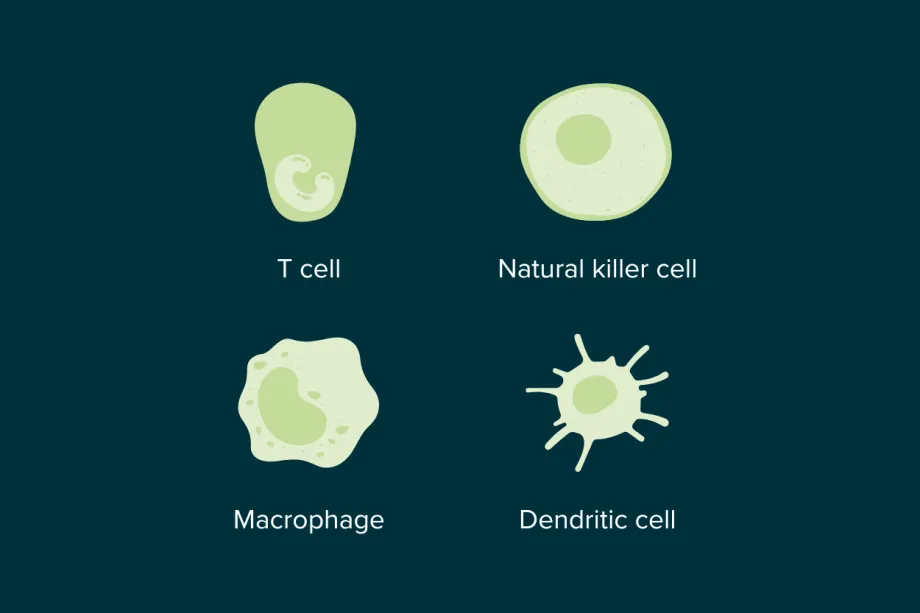
Some of the immune cell types that use cytokines to communicate.
Cytokine therapy for cancer uses artificial cytokines that mimic those found in the human body, like interferons and interleukins, to boost the body's fighting abilities. These are families of proteins that carry important signals between immune cells. For example, interleukin-2 (IL-2) can help promote the growth of white blood cells like T cells, which can attack cancer, while interferons like interferon-alpha can slow tumour growth and activate other immune cells. This type of treatment is one of the earliest approved treatments on this list, with the first treatment going into use for adults in the 1980s.
While cytokine therapy is still used today, researchers are now working on more targeted treatments. That’s because cytokines affect the whole immune system and can sometimes cause unwanted side effects. Targeted treatments aim to fight cancer more precisely, without having as much impact on healthy cells.
Monoclonal antibodies and antibody-drug conjugates
Our immune system uses tiny Y-shaped proteins called antibodies to recognise foreign molecules in the body. Our bodies can produce an amazing amount of different antibodies - up to a quintillion (1,000,000,000,000,000,000) versions! That’s because each ‘threat’ needs its own antibody, specifically shaped to recognise and lock on to it (like how a lock and key fit together) – whether it be a virus, an allergen, or a rogue cancer cell.
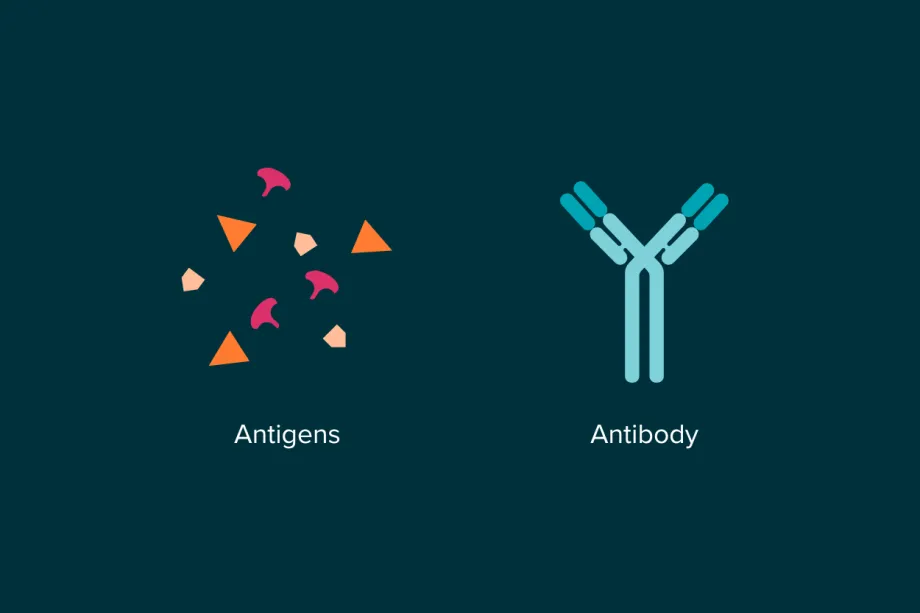
When an antibody finds and binds to a cancer-related antigen (usually a tiny molecule on the surface of a cancer cell), it flags the cell for destruction by the immune system. This natural process helps eliminate damaged or abnormal cells. However, the effectiveness of natural antibodies varies between individuals, and they don’t always target cancer cells efficiently.
That’s where monoclonal antibodies come in. They are artificial antibodies that are specifically designed to fight cancer. They can last for longer in the body, be given in the right doses, and they target cell surface antigens that are specific to cancer. This means that there are normally fewer side effects, because they don’t harm normal healthy cells as much.
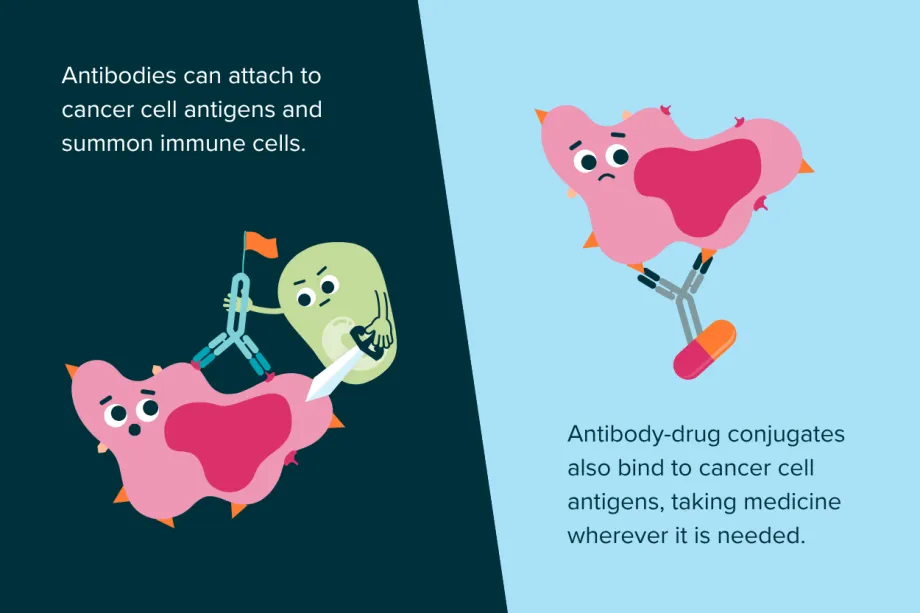
Monoclonal antibodies can also be combined with chemotherapy medicines to create something called antibody-drug conjugates. Instead of giving chemotherapy via IV or orally, which spreads all over the body and can harm healthy cells, the drug is attached to the antibody. These act like guided missiles, carrying the drug directly to cancer cells and sparing healthy ones from the toxic medicine.
Bi-specific antibodies
When antibodies flag a threat to the immune system, they can recruit immune cells like natural killer cells or macrophages to help destroy the target. However, to trigger a full T cell response, a different type of immune cell - called an antigen-presenting cell - must first capture a piece of the cancer cell and present it to the T cells. This is vital to launch the adaptive immune system's targeted attack.
That’s why scientists created bispecific T-cell engagers (BiTEs), a type of bispecific antibody. While monoclonal antibodies can only bind to one antigen, bi-specific antibodies like BiTEs can bind to two different targets at once. BiTEs bind to a cancer cell and a T cell at the same time, physically pulling them together and activating the T cell. Instead of relying on antigen presenting cells, BiTEs directly speed up and supercharge the immune system’s ability to hunt and destroy cancer.
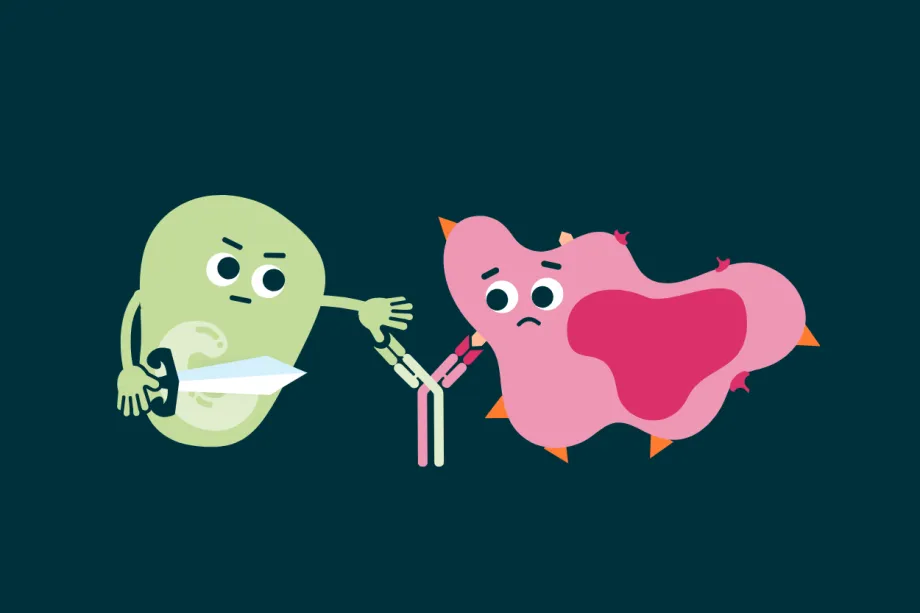
BiTEs can bring together T cells and cancer cells, speeding up the immune response.
However, this is still an emerging area of research. Scientists need to carefully choose the right antigens to target, ones found only on cancer cells and not on healthy tissue. This is harder than it sounds, because cancer cells used to be healthy cells – so they are very good at pretending to still be normal. It’s important to find the right antigens to avoid harmful side effects. And because every cancer type, subtype, and mutation can look different, scientists need to repeat this development process for each permutation of cancer.
Checkpoint inhibitors
Our immune system is powerful, so there must be checkpoints that can stop the immune response before it destroys healthy cells. T cells have proteins on their surface called checkpoint receptors, which act like brakes. When these receptors receive a ‘stop’ signal protein from other cells, they stop the T cell from attacking.
Some cancer cells take advantage of this by sending too many ‘stop’ signals using proteins like PD-L1. This prevents the T cells from killing the cancer cells. Checkpoint inhibitors are medicines that block this process. They stop the checkpoint receptors on T cells from receiving the ‘stop’ signals, allowing the T cells to stay active and continue fighting cancer.
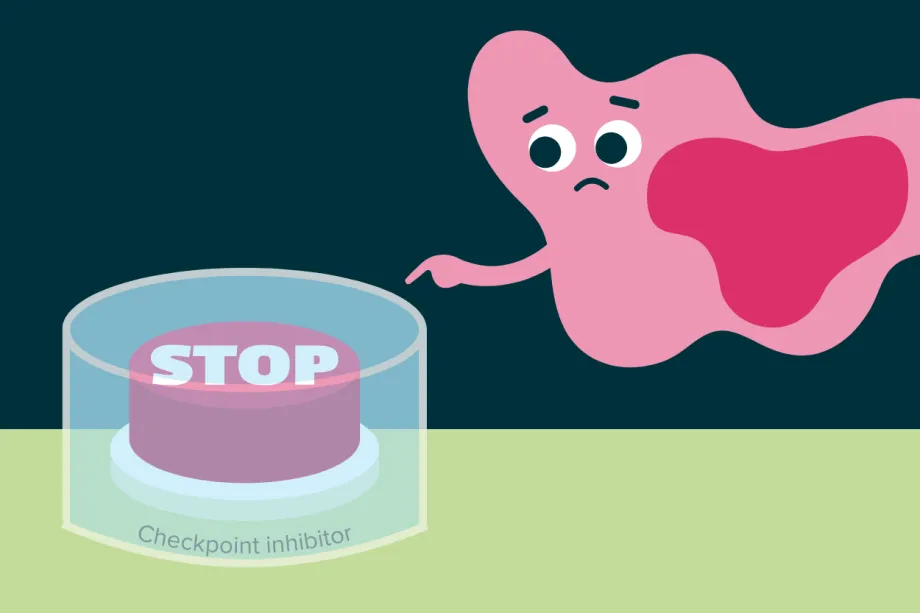
Checkpoint inhibitors physically block cancer cells from turning off T cells.
Immune checkpoint inhibitors were approved in the UK for the first time in 2012, and there are already examples like nivolumab in use for some childhood cancers. However, this type of treatment is more common for adults, so research is needed to help bring these innovations to more childhood cancer treatments.
Adoptive cell therapy (or cellular immunotherapy)
There are medicines that work inside the body to help our immune cells fight cancer better – but what if they’re still not effective enough? Scientists have developed treatments that take a patient’s immune cells out of their body, modify them to improve their cancer-fighting abilities, and return them to the patient. This is called adoptive cell therapy (or cellular immunotherapy), and usually uses T cells or another type of immune cell called a natural killer cell.
The most famous example of this is CAR T-cell therapy, which was first approved in the UK in 2019 to treat some childhood cancers. It stands for ‘Chimeric Antigen Receptor T-cell’ therapy, and it uses modified T cells to fight cancer.
The treatment is unique to each patient, taking the patient's own T cells and genetically modifying them by adding a special gene. The gene helps the T cells make a new receptor (called a CAR) which allows them to better recognise and bind to proteins on cancer cells. The modified T cells are grown in the lab so that there are millions of trained CAR T-cells. When put back into the patient, they can hunt down cancer, finding and destroying it much more easily.
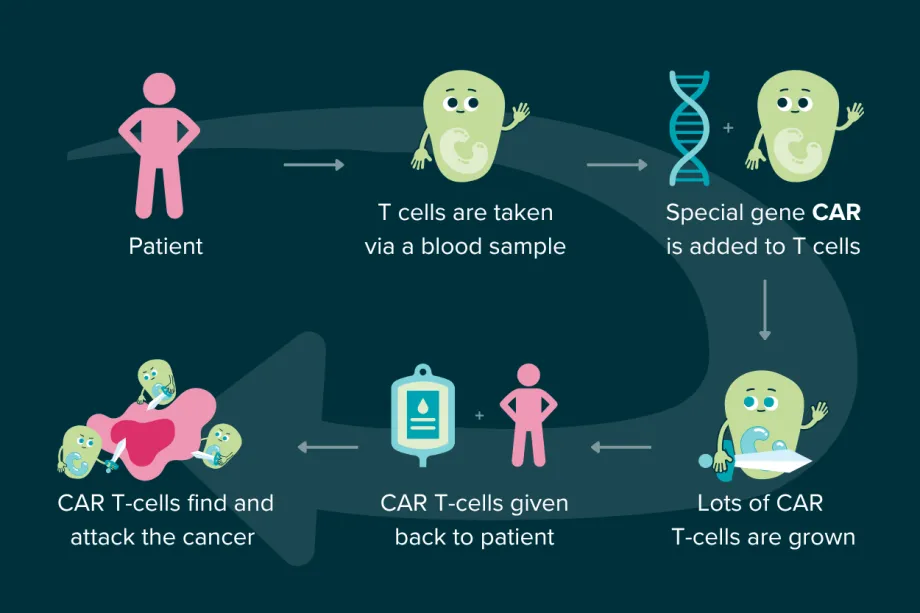
Therapeutic cancer vaccines
Vaccines normally give a healthy person antigens that the immune system needs to recognise in the future (for example, by giving inactivated versions of the flu virus to enable the immune system to fight it off faster). There are vaccines against specific viruses that are linked to adult cancers (like the human papillomavirus, which can cause cervical cancer). However, we normally think about vaccines as being preventative (or prophylactic) – so how could they help children who already have cancer?
The first therapeutic cancer vaccine was approved in 2010 for prostate cancer, following decades of research into ways to get the immune system to fight cancer better. The various types of cancer vaccines all have the same general goal: to deliver cancer-related antigens to the immune cells, boosting the natural cancer fighting abilities of the immune system. There are currently no vaccines available for childhood cancer – but researchers are working on it.
Oncolytic virus therapy
Normally, we want to avoid catching viruses – especially when dealing with other illnesses like cancer. So, it might seem a bit counterintuitive that certain viruses can help fight cancer. Viruses act like a parasite, despite not really being ‘alive’ by most definitions. They inject our cells with their own genetic code. That DNA or RNA, depending on the type of virus, is then taken up by the cell and the cell is forced to become a virus-making machine. Eventually the cell will have made so many viruses that it explodes – releasing many more viruses to go and infect other cells. Obviously, that’s quite bad if the virus is infecting healthy cells – but what if it targeted cancer cells?
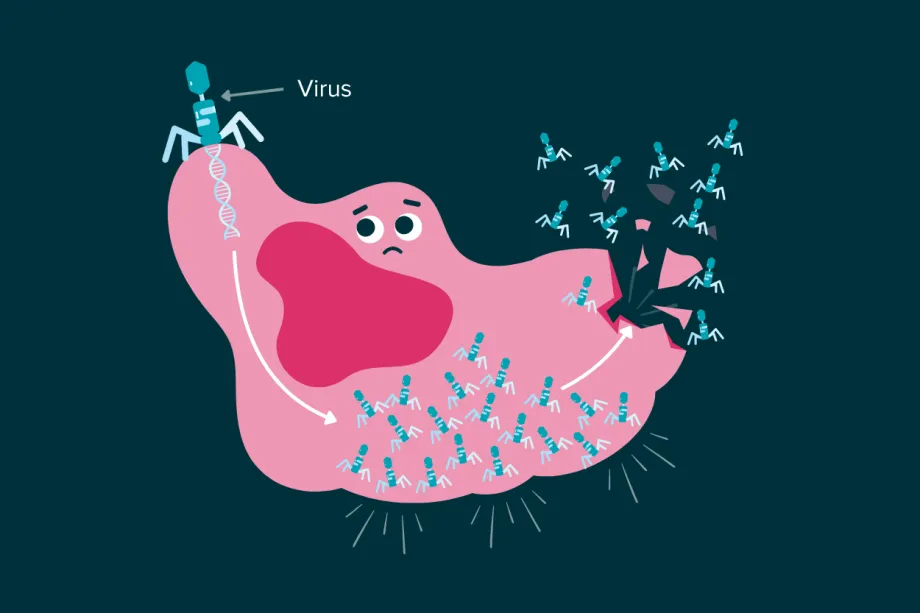
Some viruses can force cancer cells to make so many new viruses that the cell explodes.
Oncolytic virus therapy is a type of treatment that uses viruses to target and destroy cancer cells. These viruses are often engineered to be better at infecting cancer cells, in the hopes of reducing the effects of an infection on healthy cells. When oncolytic viruses infect cancer cells, it fights cancer in two ways. The first is by killing that cell – but when the cell bursts to release all the new viruses, it also releases a lot of cancer cell antigens. This boosts the immune response, killing the cancer even faster.
There is only one approved version of this treatment in the UK at the moment, for advanced skin cancer, but researchers are working on versions for other types of cancer.
The future of immunotherapy
Immunotherapy is already changing the way we treat cancer, and it’s only just getting started. While some types are already saving lives, others are still in the early stages of research. Between CCLG and The Little Princess Trust, our researchers are working on new adoptive cell therapies, antibody-drug conjugates, oncolytic virus therapies and many more.
As scientists learn more about how the immune system works, and how cancer hides from it, we hope that children, teenagers and young adults with cancer will see more and more safe, effective, and targeted treatments. We won’t stop funding research until every young patient, regardless of cancer type, can survive their cancer.

Ellie Ellicott is CCLG’s Research Communication Executive.
She is using her lifelong fascination with science to share the world of childhood cancer research with CCLG’s fantastic supporters. You can find Ellie on X: @EllieW_CCLG




